PI: Ricardo Ibarra/Jesús Martínez de la Fuente
Nanosci-ERA+, ERANET
(2009-2012)
RNA interference via the use of small-interfering RNA (siRNA) is a powerful and useful tool to block gene function through sequence-specific post-transcriptional gene silencing, playing an important role in downregulation of gene expression. siRNAs can be transfected into mammalian cells by a variety of methods that influence the strength and duration of the silencing response, which in turn is affected by the amount of siRNA effectively delivered and by the potential of each siRNA to suppress its target. Nevertheless, naked siRNAs show extremely short half-lives due to RNases activity, poor chemical stability, and dissociation from vector. In fact, the major obstacle to clinical application is the uncertainty about how to deliver siRNA with maximal therapeutic impact.
Nanotechnology in general, and nanoparticles in particular, offer unprecedented opportunities towards effective cancer therapy, as new multifunctional devices capable of bypassing biological barriers for the targeted delivery of therapeutic agents can be easily assembled, reducing systemic toxicity and severe adverse side effects avoiding the undesirable distribution to healthy organs and tissues. The main challenge remains the development of systems for molecular therapy capable of circulating in the blood stream undetected by the immune system and being recognized by the desirable target and signalized it for effective delivery of the therapeutic agents. Nanoparticles exhibit excellent physic-chemical properties for application in drug delivery, namely their reduced sizes, rendering them capable of interacting with biomolecules in a one-to-one scale, and the high surface to volume ratio allowing for surface functionalisation with a plethora of molecules, for specific targeting and drug payloads. Among the numerous nanoparticle formulations designed to improve siRNA delivery and efficiency, those based on AuNPs have been extensively investigated without undesirable immune response or off-target effects. To our knowledge, with the exception of one report on AuNPs for systemic administration of siRNA in humans, over the last six years, formulations using AuNP-siRNA have solely been tested in cell cultures targeting reporter genes, such as luciferase or green fluorescence protein. However, as with any drug treatment, gene therapy vehicles need extensive testing in archetypal animal models before translation into the clinics, and this requires the coordinated effort of interdisciplinary research groups.
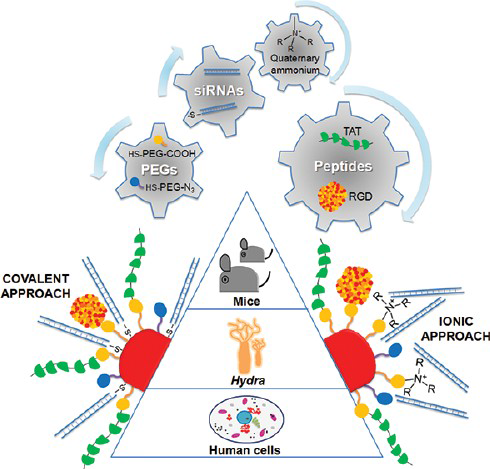
Here we used a functional comparative approach to assist the smart design, synthesis and testing of a library of multifunctional nanodevices for RNAi, resulting in a nanocarrier based on AuNPs as delivery system, with demonstrated functionality both in vitro and in vivo for RNAi-based therapy. Several biochemical moieties, cell penetrating and cell adhesion peptides were selected to enhance cellular recognition and uptake and confer specific downregulation of gene expression. The c-myc protooncogene, a key gene playing a pivotal role in cell cycle and tissue homeostasis, was selected as RNAi target; poly(ethylene glycol) (PEG) molecules were incorporated to guarantee nanoparticle stability and avoid unspecific interactions and three in vitro/in vivo model systems were selected: human cells (HeLa), freshwater polyps (Hydra vulgaris) and mouse (C57BL/6j). This chemical and biological strategy allowed us to compare the effect of the siRNA linkage to the NPs and the effect of cell penetration and adhesion peptides, finally leading to an optimally designed nanocarrier for RNAi therapeutic purposes.